Relative Configuration of flexible Molecules determined by RDC-enhanced NMR.
Results shown
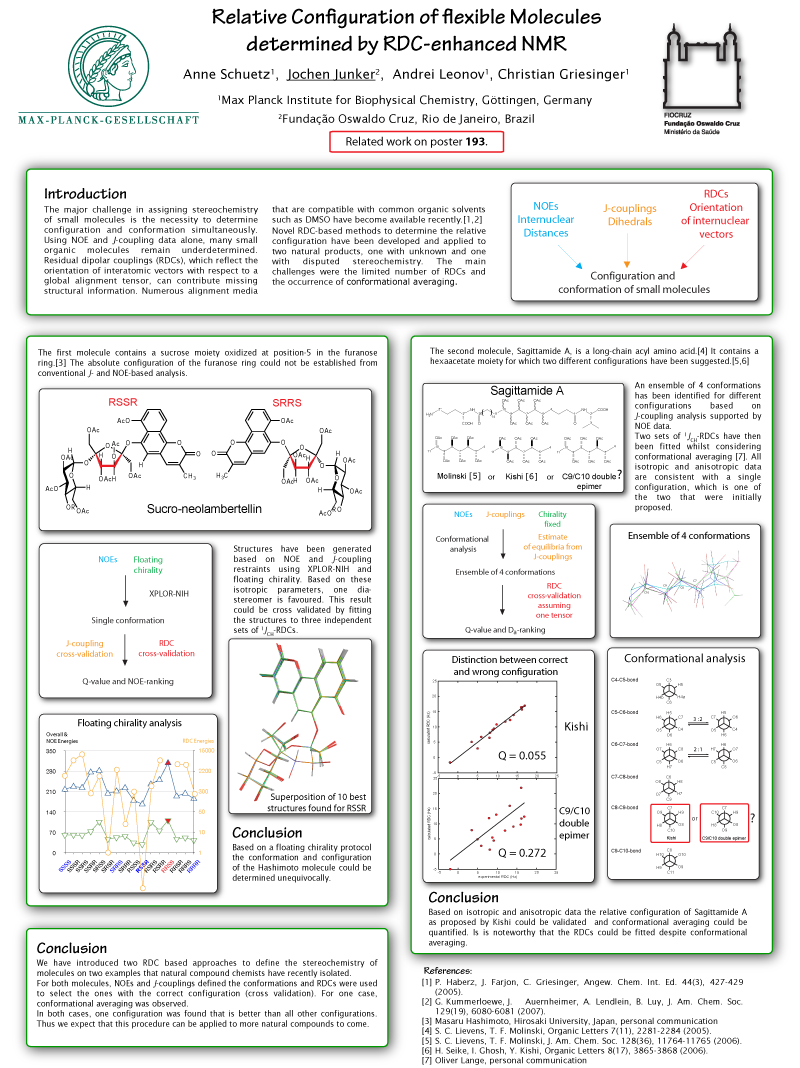
The poster shows the use of RDCs (residual dipolar couplings) for the determination of the relative stereochemistry of two natural products. Several alignment media for organic solvents were used to obtain different sets of RDCs. A new X-Plor protocol was developed that is capable of using this data in order to filter out the configurations that best fit the RDCs of a set of conformations generated using floating chirality.
Literature cited
The following literature references are used for this poster:
- P. Haberz, J. Farjon, C. Griesinger, Angew. Chem. Int. Ed. 44(3), 427-429 (2005).
- G. Kummerloewe, J. Auernheimer, A. Lendlein, B. Luy, J. Am. Chem. Soc. 129(19), 6080-6081 (2007).
- Masaru Hashimoto, Hirosaki University, Japan, personal communication
- S. C. Lievens, T. F. Molinski, Organic Letters 7(11), 2281-2284 (2005).
- S. C. Lievens, T. F. Molinski, J. Am. Chem. Soc. 128(36), 11764-11765 (2006).
- H. Seike, I. Ghosh, Y. Kishi, Organic Letters 8(17), 3865-3868 (2006).
- Oliver Lange, personal communication
The Poster
 English
English
 Deutsch
Deutsch
 Português
Português